Regra do Octeto
2 participantes
Página 1 de 1
 Regra do Octeto
Regra do Octeto
A regra do octeto afirma que os gases nobres são o modelo de estabilidade por apresentarem 8 elétrons na camada de valência, se apresentando como inertes nas condições ambientes. Mas, por que isso acontece ? Por que exatamente são necessários 8 elétrons na camada de valência (ou 2 no caso do hélio) para que um elemento apresente estabilidade ? Por que não 6 elétrons na camada de valência, por exemplo ????

miguelito3,1415926- Iniciante
- Mensagens : 25
Data de inscrição : 03/04/2024
Idade : 19
 Re: Regra do Octeto
Re: Regra do Octeto
miguelito3,1415926, eu achei interessante essa parte do TEXTO indicado abaixo: "Um octeto completo é muito estável porque todos os orbitais estarão cheios. Átomos com maior estabilidade possuem menos energia, portanto uma reação que aumenta a estabilidade dos átomos liberará energia na forma de calor ou luz.", ou seja, OCTETO COMPLETO = ORBITAIS PREENCHIDOS = maior estabilidade = menor energia enquanto que OCTETO INCOMPLETO = ORBITAIS SEMI-PREENCHIDOS = menor estabilidade = maior energia:miguelito3,1415926 escreveu:A regra do octeto afirma que os gases nobres são o modelo de estabilidade por apresentarem 8 elétrons na camada de valência, se apresentando como inertes nas condições ambientes. Mas, por que isso acontece ? Por que exatamente são necessários 8 elétrons na camada de valência (ou 2 no caso do hélio) para que um elemento apresente estabilidade ? Por que não 6 elétrons na camada de valência, por exemplo ????
The Octet Rule
- Wikipedia
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. When discussing the octet rule, we do not consider d or f electrons. Only the s and p electrons are involved in the octet rule, making it useful for the main group elements (elements not in the transition metal or inner-transition metal blocks); an octet in these atoms corresponds to an electron configurations ending with s2p6
Introduction
In 1904, Richard Abegg formulated what is now known as Abegg's rule, which states that the difference between the maximum positive and negative valences of an element is frequently eight. This rule was used later in 1916 when Gilbert N. Lewis formulated the "octet rule" in his cubical atom theory. Atoms will react to get in the most stable state possible. A complete octet is very stable because all orbitals will be full. Atoms with greater stability have less energy, so a reaction that increases the stability of the atoms will release energy in the form of heat or light.
Octet Rule
A stable arrangement is attended when the atom is surrounded by eight electrons. This octet can be made up by own electrons and some electrons which are shared. Thus, an atom continues to form bonds until an octet of electrons is made.
Normally two electrons pairs up and forms a bond, e.g., H2
For most atoms there will be a maximum of eight electrons in the valence shell (octet structure), e.g., CH4
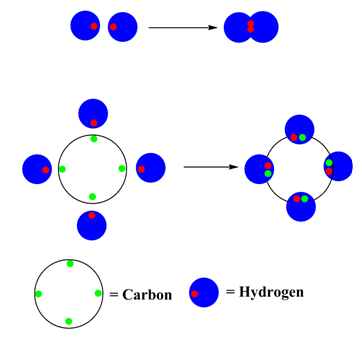
Figure 1: Bonding in H2 and methane ( CH4 )
The other tendency of atoms is to maintain a neutral charge. Only the noble gases (the elements on the right-most column of the periodic table) have zero charge with filled valence octets. All of the other elements have a charge when they have eight electrons all to themselves. The result of these two guiding principles is the explanation for much of the reactivity and bonding that is observed within atoms: atoms seek to share electrons in a way that minimizes charge while fulfilling an octet in the valence shell.
Note
The noble gases rarely form compounds. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their configuration. All other elements attempt to gain, lose, or share electrons to achieve a noble gas configuration.
Example 1: NaCl Salt
The formula for table salt is NaCl. It is the result of Na+ ions and Cl- ions bonding together. If sodium metal and chlorine gas mix under the right conditions, they will form salt. The sodium loses an electron, and the chlorine gains that electron. In the process, a great amount of light and heat is released. The resulting salt is mostly unreactive — it is stable. It will not undergo any explosive reactions, unlike the sodium and chlorine that it is made of. Why?
Solution
Referring to the octet rule, atoms attempt to get a noble gas electron configuration, which is eight valence electrons. Sodium has one valence electron, so giving it up would result in the same electron configuration as neon. Chlorine has seven valence electrons, so if it takes one it will have eight (an octet). Chlorine has the electron configuration of argon when it gains an electron.
The octet rule could have been satisfied if chlorine gave up all seven of its valence electrons and sodium took them. In that case, both would have the electron configurations of noble gasses, with a full valence shell. However, their charges would be much higher. It would be Na7- and Cl7+, which is much less stable than Na+ and Cl-. Atoms are more stable when they have no charge, or a small charge.
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Electronic_Structure_of_Atoms_and_Molecules/Electronic_Configurations/The_Octet_Rule
Última edição por Jigsaw em Sáb 06 Abr 2024, 11:26, editado 1 vez(es) (Motivo da edição : readequação do texto da mensagem)

Jigsaw- Monitor

- Mensagens : 772
Data de inscrição : 26/12/2020
Localização : São Paulo/SP
miguelito3,1415926 gosta desta mensagem
 Re: Regra do Octeto
Re: Regra do Octeto
Isso é belo ! Valeu, meu caro.

miguelito3,1415926- Iniciante
- Mensagens : 25
Data de inscrição : 03/04/2024
Idade : 19
 Tópicos semelhantes
Tópicos semelhantes» PUC PR - Regra do octeto
» regra do octeto
» regra do octeto
» Regra do Octeto
» Excessão a regra do octeto
» regra do octeto
» regra do octeto
» Regra do Octeto
» Excessão a regra do octeto
Página 1 de 1
Permissões neste sub-fórum
Não podes responder a tópicos












